About the USC TCORS
Since 2013, the USC Tobacco Center of Regulatory Science (TCORS) has generated vital research that informs FDA policies aimed at reducing youth exposure to harmful tobacco and nicotine products. During its first funding cycle (2013–2018), USC TCORS was among the first to document the harms of e-cigarette use among adolescents and young adults (AYAs), including evidence of escalating use patterns and the increased risk of transitioning to combustible tobacco. In its second cycle (2018–2023), the center advanced understanding of how factors such as flavors, nicotine concentration, and product design enhance the appeal, uptake, and addictive potential of e-cigarettes. Our team also provided early evidence on the emerging impact of oral nicotine pouches (ONPs) on youth.
Now in its third cycle (2023–2028), USC TCORS is addressing the FDA’s newest regulatory challenges related to the rapidly evolving non-combustible oral nicotine market. The center’s current work focuses on identifying which ONPs, e-cigarettes, and future non-combustible products—and their marketing strategies—most increase risk among AYAs, and how these effects vary across different populations. Through this ongoing research, USC TCORS continues to provide scientific evidence that supports effective regulation and protects youth from tobacco-related harms.

About the Tobacco Centers for Regulatory Science
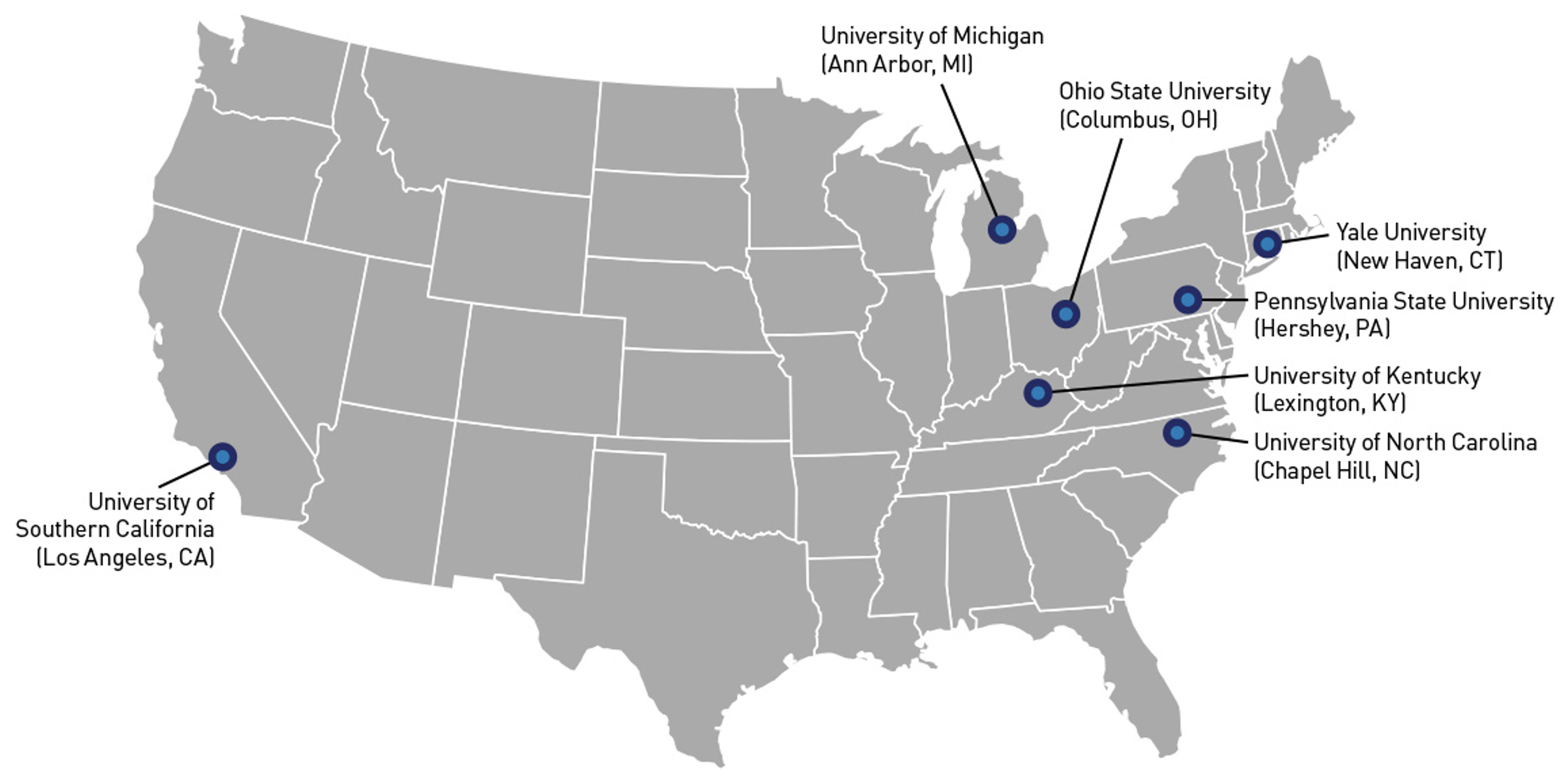
In partnership with the FDA and National Institutes of Health (NIH), seven Tobacco Centers of Regulatory Science (TCORS) were awarded new grants in September 2023 to continue advancing tobacco research. This ongoing collaboration brings together leading scientists to generate evidence that helps guide the FDA’s efforts to regulate tobacco products and protect public health. Through the TCORS program, researchers provide vital insights that inform current and future tobacco policies, while also maintaining the flexibility to address new challenges as the tobacco marketplace evolves.
Primary Research Projects
Our Center is built around four interconnected research projects that examine how modern oral nicotine products (ONPs) and e-cigarettes affect tobacco use among adolescents and young adults. We study how these products influence the initiation, escalation, and potential for addiction, as well as patterns of using multiple tobacco products.
In addition to tracking behavior across diverse populations, we also investigate which product features and marketing strategies increase the likelihood of youth use. This work helps identify key factors that may contribute to increased risk—providing valuable insights to inform tobacco regulation and protect public health.

Project 1: Use of novel non-combustible tobacco products among US youth
This study looks at how the availability of new non-combustible tobacco products affects use among U.S. youth. In partnership with the Monitoring the Future (MTF) Study, we are adding new questions to the annual survey of 8th, 10th, and 12th graders to track trends in youth use of these products from 2024–2028. We also aim to understand which product types, features, and population groups are driving these trends.
Project Lead(s): Adam Leventhal, PhD
Project 2: Longitudinal patterns of non-combustible product use in AYA
This long-term study follows regional groups of youth and young adults ages 14 to 22 to understand how and when they start using different tobacco and nicotine products. We’re tracking patterns of oral nicotine product, e-cigarette, and combusted tobacco use—including initiation, escalation, and transitions to using multiple products. The study also identifies which product features and population groups are linked to these changing patterns of use.
Project Lead(s): Jessica Barrington-Trimis, PhD and Chanita Hughes-Halbert, PhD
Project 3: Abuse liability and appeal of ONPs in young adults
This study explores which features of oral nicotine products (ONPs) make them more appealing and potentially more addictive to young adult vapers. We’re working with a nationwide group of e-cigarette users ages 21–25 who are not planning to quit vaping and are open to trying ONPs. Using an innovative virtual study visit approach, we test how different product types, flavors, and nicotine levels affect both product appeal and potential for misuse.
Project Lead(s): John Monterosso, PhD and Raina Pang, PhD
Project 4: Social media influences on engagement and susceptibility to non-combustible use
This study examines public social media posts and conducts a novel experiment with youth to identify which marketing strategies for flavored non-combustible products are most engaging and may increase susceptibility among young people.
Project Lead(s): Jennifer Unger, PhD
Career Enhancement Core
The USC-TCORS Career Enhancement Core is dedicated to developing the next generation of tobacco regulatory science researchers. Our programs provide career-building opportunities that integrate deep scientific expertise with a strong understanding of regulatory issues. We oversee the Pilot Research Program, which supports junior investigators as they pursue independent research aligned with our Center’s integrative theme. In addition, we offer resources and mentorship to help postdoctoral scholars expand their professional networks and gain visibility within the broader Tobacco Regulatory Science (TRS) community.
Core Lead(s): Caryn Lerman, PhD and Raina Pang, PhD
Measures and Materials Core
The Measures and Materials Core (MMC) applies innovative and rigorous research methods to develop, refine, validate, and evaluate assessments and materials that support USC TCORS projects and contribute to the broader network of tobacco regulatory science.
MMC gathers insights from key data sources to keep USC TCORS informed of emerging trends in the tobacco marketplace, policy environment, and population needs. In collaboration with TCORS investigators, MMC designs and implements measures and materials that enhance research impact and ensure relevance across diverse communities.
Core Lead(s): Matthew Kirkpatrick, PhD
Data Processing and Analysis Core
The Data Processing and Analysis Core (DPAC) ensures that USC TCORS research produces high-quality, policy-relevant findings to protect adolescents, young adults, and other groups targeted by the tobacco industry. DPAC provides comprehensive data support across all USC TCORS projects—from designing efficient data collection systems to ensuring data quality, managing processing workflows, and developing robust analysis plans. By offering expertise in study design, statistics, and analytics, DPAC helps strengthen every stage of research and ensures that USC TCORS findings are timely, reliable, and impactful.
Core Lead(s): Ming Li, PhD and Junhan Cho, PhD
Cores
Our Center includes four cores that optimize the productivity and impact of our regulatory science.

Featured Publications
Nicotine Concentration of E-Cigarettes Used by Youths
Prevalence of Nicotine Pouch Use Among US Adults
Association of Race-Ethnicity Intersection With Disparities in Cigarette Smoking in U.S. Adults
Assessing the public discourse on Twitter: Reactions to the JUUL e-cigarettes ban in the United States
Disposable E-Cigarette Use and Subsequent Use Patterns in Adolescents and Young Adults
Nicotine-cannabis transitions and nicotine abstinence among United States adults
Effects of a fruit-ice combination flavor on appeal and sensory experience of vaping and moderation by preexisting e-cigarette flavor preference
Oral Nicotine Product Use and Vaping Progression Among Adolescents
Effect of packaging with versus without candy-oriented marketing themes on the appeal and sensory attributes of flavoured e-cigarettes
Ice flavours and non-menthol synthetic cooling agents in e-cigarette products: a review
Appeal and Sensory Characteristics of Oral Nicotine Products in Young Adults Who Vape E-Cigarettes
Effects of 'Ice' flavoured e-cigarettes with synthetic cooling agent WS-23 or menthol on user-reported appeal and sensory attributes
Prospective study of e-cigarette use and respiratory symptoms in adolescents and young adults
Vaping Devices with Video Games. Subst Use Misuse
Impact of Instagram and TikTok influencer marketing on perceptions of e-cigarettes and perceptions of influencers in young adults: a randomised survey-based experiment
US adolescents' response to nicotine warning labels in influencer e-cigarette marketing social media posts: a survey-based randomised between-subject experiment
Young Adults' Exposure to and Engagement With Tobacco-Related Social Media Content and Subsequent Tobacco Use
Effects of flavour and modified risk claims on nicotine pouch perceptions and use intentions among young adults who use inhalable nicotine and tobacco products: a randomised controlled trial
Effects of exposure to snus marketing with versus without modified risk tobacco product claims on snus use intention and perceived harm among young adults
Our Team
Directors & Administrative Staff

Adam M. Leventhal, Ph.D.
TCORS Admin Core, Co-Lead; Project 1, Lead
Keck School of Medicine

Caryn Lerman, Ph.D.
TCORS Admin Core, Co-Lead; Career Enhancement Core, Co Lead
Keck School of Medicine


Project and Core Leads / Co-Investigators

Dayoung Bae, Ph.D.
Data Processing and Analysis Core, Co-Investigator
USC Keck School of Medicine


David Birtwell
Data Processing and Analysis Core, Co-Investigator
USC Keck School of Medicine

Junhan Cho, Ph.D.
TCORS Project 3, Co-Lead; Career Enhancement Core, Co-Lead
USC Keck School of Medicine


Sandrah Eckel, Ph.D.
Data Processing and Analysis Core, Co-Investigator
USC Keck School of Medicine








Raina Pang, Ph.D.
TCORS Project 3, Co-Lead; Career Enhancement Core, Co-Lead
USC Keck School of Medicine

John Patrick-Allem, Ph.D.
Measures and Materials Core (MMC), Co-Investigator
Rutgers University

Louisiana Sanchez, Ph.D.
Post-doctoral Scholar, TCORS Trainee
USC Keck School of Medicine CORS Project 3, Co-Lead
USC Dornsife


Robin Stevens, Ph.D.
Measures and Materials Core, Co-Investigator
USC Annenberg School for Communication and Journalism

Steve Sussman, Ph.D., FAAHB, FAPA, FSPR
Project 1, Co-Investigator
USC Keck School of Medicine

Since its inception, the Institute has successfully mobilized and expanded membership to over 80 faculty from 10 different schools, colleges, and hospitals.
RESEARCH GROUPS AND COMMITTEES

PUBLICATIONS

NOTABLE FINDINGS

PILOT GRANT PROGRAM

RESEARCH FACILITIES

PROGRAMMATIC UNITS
We are advancing science, treatment, and policy to improve the lives of those touched by addiction.
The USC Institute for Addiction Science is the nation’s first university-wide transdisciplinary addiction science institute. We aim to break down silos that have historically prevented scholars and practitioners from different disciplines from working together. Join us!


